Davy safety lamp
Add to My Folder
Combine coverage of the History and Science programmes of study with this intriguing article.
Biography
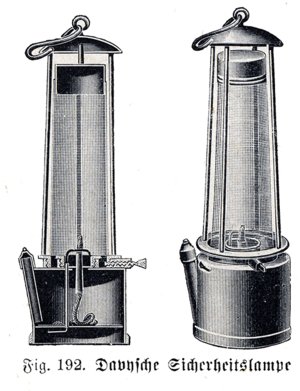
Humphry Davy was born in Cornwall in 1778 and worked first as a chemist in Penzance. Later he moved to London to become assistant lecturer in chemistry at the Royal Institution, where his talks on science became very popular with members of the public. Davy, who was also a keen fisherman and artist, discovered a number of important chemicals including calcium, sodium, potassium, magnesium and chlorine. He became a member of the Royal Society in 1803 and was its President between 1820 and 1827. He was knighted for his services to science in 1812 and became a baronet in 1818. Davy died in Geneva, Switzerland in 1829.
Published 29 June 2016
Reviews
You need to be signed in to place a review.